Key learning points
- Portal vein thrombosis can present in the acute or chronic setting in patients with or without cirrhosis
- The cornerstone of therapy is anticoagulation, which is mandatory where there is intestinal ischaemia or an underlying pro-coagulant condition
- Vitamin K antagonists and heparin are recommended, whereas direct oral anticoagulants should be used only in the context of research
Introduction
There is considerable heterogeneity in the clinical presentation and course of portal vein thrombosis (PVT). It is useful to differentiate between acute and chronic PVT (either cirrhotic or non-cirrhotic) based on clinical presentation (Table 1).1–3
Acute PVT
Acute PVT is associated with local (e.g. pancreatitis) and/or systemic prothrombotic conditions (e.g. myeloproliferative neoplasm, antiphospholipid syndrome, paroxysmal nocturnal haemoglobinuria, and, occasionally, inheritable thrombophilia). Patients may present with symptoms of the precipitating condition (e.g. malignancy, pancreatitis) with PVT being found incidentally on ultrasound scans. Alternatively, presentation might be recurrent or severe abdominal pain, suggesting venous ischaemia of the gut and extension of the thrombus to the superior mesenteric vein (SMV). Due to compensatory increase in hepatic arterial blood flow, liver function is usually unaltered even in extensive acute PVT.
Chronic PVT
Chronic PVT generally occurs in the setting of cirrhosis. The annual incidence is estimated to be 10–15%. It commonly presents asymptomatically on routine surveillance ultrasound scans or as new-onset or worsening decompensation. The pathophysiological characteristics are reduced portal flow, haemostatic imbalance (elevation in factor VIII and decrease in natural anticoagulants, such as protein C, protein S or antithrombin III) and endothelial injury (elevated von Willebrand factor and circulating endothelial microparticles). All patients with chronic PVT must be assessed for hepatocellular carcinoma.1,2
Unrecognised acute PVT can lead to chronic PVT. In the absence of cirrhosis, chronic PVT is an important cause of portal hypertension in children from developing parts of the world. Umbilical sepsis and intra-abdominal infection are precipitant in a small number of children. These children present with well-tolerated variceal bleeds and massive splenomegaly with hypersplenism. Varying severity of portal cavernoma and portal biliopathy can eventually develop in children with long-standing PVT due to biliary-tree obstruction secondary to peri-choledochal varices.1
Classifying PVT as anatomical (Table 2)4 or an anatomic-functional (site of PVT, degree of occlusion, duration and presentation, extent of PV system involvement and type and presence of underlying liver disease) may help in establishing prognosis and in overall management decision making.5
Diagnosis
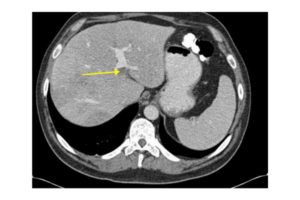
Doppler ultrasonography of the abdomen is usually the initial test to diagnose PVT (Table 1). Cross-sectional imaging, such as multiphase contrast-enhanced CT (Table 1, Figure 1) and MR cholangiography, helps in delineating the extent, acuity and often the local cause of PVT (hepatocellular carcinoma, pancreatitis, source of intra-abdominal sepsis, intestinal infarction, portal biliopathy). Most patients will benefit from an evaluation for systemic prothrombotic conditions, and the extent should be individualised and guided by clinical presentation.1,2
Treatment
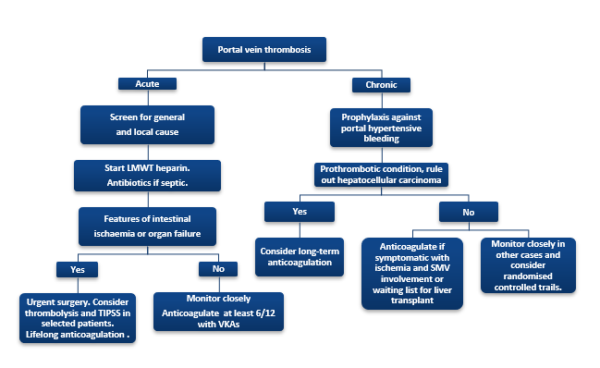
Anticoagulation and management of any predisposing factor(s) remain the mainstay of management of PVT (Figure 2). The management of complications of PVT (e.g. intestinal ischemia and portal biliopathy) and role of advanced therapies, such as placement of a trans-jugular intrahepatic portosystemic shunt (TIPSS) and surgery, are beyond the scope of this article. Anticoagulation is mandatory in patients with symptomatic PVT (intestinal ischemia) or a pro-coagulant condition.
Anticoagulants
Spontaneous resolution of acute symptomatic non-cirrhotic PVT is highly unlikely and immediate institution of anticoagulation is recommended.1 The treatment duration is usually 6 months if a solely transient local cause is identified, but may have to be extended in absence of an identifiable local cause and/or identification of a persisting systemic cause.2
In patients with cirrhosis, anticoagulation can be considered in symptomatic patients with extension of the clot to the SMV or a definite pro-coagulant risk factor. Despite limited evidence, anticoagulation according to the European Association for the Study of the Liver (EASL) guidelines2 and Baveno VI consensus3 may be used to support cirrhotic PVT patients while on the transplant waiting list. PVT progression can increase the complexity of surgery and adversely affect the post-transplant outcomes. The risks and benefits of anticoagulation in other settings remain unclear.
In patients with non-cirrhotic chronic PVT, current EASL and Baveno VI recommendations support indefinite anticoagulation.2,3 This approach might not be applicable in patients of Asian origin, as very limited benefit from non-selectively anticoagulating these patients is reported.6
In all patients with chronic PVT, assessment and institution of adequate prophylaxis for gastroesophageal varices are recommended prior to anticoagulation.7
Are anti-coagulants effective?
For acute PVT, limited data suggest that prompt anticoagulation effectively prevents thrombus extension and can result in recanalisation in 38–45% of patients.8 Direct thrombolysis (via TIPSS) may be useful. A few data suggest a high rate of immediate recanalisation and good long-term patency.9 In a recent UK study of patients with non-cirrhotic acute PVT with intestinal ischaemia, recanalisation was reported in 86% of 22 patients with a stepwise approach of systemic thrombolysis and local clot dissolution therapy by TIPSS and thrombolysis.10
Retrospective studies have shown that anticoagulants are efficacious in achieving recanalisation and prevention of de-novo thrombotic events in patients with chronic non-cirrhotic PVT, without a substantial increase in bleeding complications.11
In chronic cirrhotic PVT, patients might experience spontaneous complete recanalisation. Studies have demonstrated anticoagulation to be safe and effective in increasing the rate of recanalisation (72% versus 42% without anticoagulation), but overall survival is not altered.12
In a highly selected group of patients, use of anticoagulants is not associated with significantly increased bleeding risks. An adequate platelet count (>50,000 cells/mm3 or 50 × 109/L) and prophylaxis for gastroesophageal varices is recommended at the time of initiation.1–3,7
In a single randomised trial, anticoagulation with low-molecular-weight heparin (LMWH) in patients with advanced cirrhosis was efficacious in preventing PVT and reducing the risk of worsening decompensation.13
Which anticoagulants are safe and effective?
There is lack of clarity about the choice of anticoagulants in the literature. In clinical practice, most patients who warrant anticoagulation are initiated on LMWH or unfractionated heparin and overlapped to vitamin K antagonists (VKAs) to maintain a target international normalisation rate of 2–3.
Direct oral anticoagulants (DOACs) are an oral alternative to VKAs. These drugs have a quicker onset of action and lower risk of bleeding, obviating the need for monitoring of international normalisation rate (Table 3). DOACs have equivalent efficacy to VKAs in treating deep-vein thrombosis and pulmonary embolism and in stroke prevention in patients with atrial fibrillation. However, patients with cirrhosis have been excluded from most trials due to imbalanced haemostasis. Although all DOACs undergo hepatic elimination to a variable extent, studies have shown them not to be associated with a significant risk of liver injury.14 DOACs can be used for licensed indications in Child’s A disease, but are contraindicated in Child B and C cirrhosis.
In small retrospective studies, the use of DOACs was found to be safe and efficacious (equivalent to VKAs) in PVT, without an increase in overall bleeding events.15,16 A randomised study in 80 patients with compensated cirrhotic PVT related to hepatitis C virus (secondary to splenectomy) showed that rivaroxaban was associated with significantly better results for recanalisation than warfarin (85% versus 45%).17 Survival was improved and the risk of bleeding complications was greatly reduced. The study was limited by small sample size and its inclusion only of post-splenectomy patients. Larger studies are warranted before strong recommendation can be made regarding the use of DOACs in the setting of cirrhosis.
It is the opinion of the authors that the use of DOACs in PVT should be restricted to research, as current evidence is lacking in quality and quantity.
Course and prognosis
Spontaneous resolution is common in patients with recent PVT, especially if it is non-occlusive. These patients are typically asymptomatic and resolution is usually demonstrated in the setting of ultrasound surveillance of cirrhosis.1
The potential impact of PVT on worsening of portal hypertension remains a debatable point. A randomised study demonstrated that PVT recanalisation had a beneficial effect on hepatic decompensation.11 By contrast, a large multicentre study found no association between recent PVT and hepatic decompensation.18
In cirrhosis, PVT reflects the severity of liver disease, and its impact on overall outcome is difficult to assess.
Patients with non-cirrhotic chronic PVT typically survive long-term with a clinical course characterised by variceal bleeding, portosystemic encephalopathy and portal cavernoma with biliopathy, which can present with recurrent cholangitis and eventually biliary cirrhosis.
Conclusions
PVT can present in the acute or chronic setting in patients with or without cirrhosis. The cornerstone of therapy is anticoagulation, which is mandatory if the patient has intestinal ischaemia or an underlying pro-coagulant condition. The role of anticoagulation in the setting of cirrhosis is debatable, but is generally recommended for patients who are on the transplant waiting list and have proven PVT. VKAs and heparin are recommended, whereas DOACs should only be used in the context of research.
Table 1. Key features differentiating acute from chronic PVT at index presentation
| Parameters | Acute PVT | Chronic PVT | |
| Clinical | Presentation | 1. Incidental as part of the inciting illness (e.g. acute pancreatitis) 2. Ischemic bowel (if mesenteric veins are involved) | 1.Usually asymptomatic 2. Worsening of liver decompensation (ascites, variceal bleeding, hepatic encephalopathy) 3. Cholangitis if there is biliopathy. |
| Precipitants | 1. Usually local and/or systemic precipitants are present | 1. Cirrhosis, being a low flow state, is a risk factor 2. Hepatocellular carcinoma needs to be ruled out | |
| Radiological | Doppler ultrasonography | Absence of flow | Multiple leash of collateral vessels (cavernoma) replacing the portal vein. |
| CT of the abdomen | Can identify precipitant and extent | · Usually required to rule out hepatocellular carcinoma (if cirrhotic) · Portal biliopathy |
Table 2. Anatomical grading of PVT4
| Grade of PVT | Description
|
| Grade I | Partial PVT (<50% of the lumen) |
| Grade II | >50% PVT (includes total occlusion) |
| Grade III | Complete PVT and proximal SMV thrombosis (distal SMV is patent) |
| Grade IV | Complete PVT and entire SMV thrombosis |
Table 3. Anticoagulant options in PVT
| Agents | Mechanism | Frequency and route of administration | Monitoring | Cautions | Liver dysfunction | Reversal agent | Comments |
| Unfractionated Heparin | Potentiate anti-thrombin | Continuous IV infusion/3-4 times daily IV/ subcutaneous injection | aPTT | Renal failure, heparin-induced thrombocytopenia | Dose adjusted according to aPTT | Protamine sulphate | Unsuitable for maintenance |
| Low molecular weight Heparin (e.g Enoxaparin) | Potentiate antithrombin and inactivate factor Xa | Once or twice daily, subcutaneous injection | Factor Xa (optional) | Renal failure | No dose adjustment | Protamine sulphate (may be partially effective) | Use supported by literature, difficult for maintenance |
| VKAs (e.g. Warfarin) | Inhibit synthesis of vitamin k dependent factors | Once daily, oral | PT or INR (narrow therapeutic range) | Unpredictable – requires close monitoring, Difficult to monitor if baseline INR is prolonged | Dose to maintain target INR | Vitamin K, prothrombin complex concentrate | Use supported by literature |
| Direct anti-thrombin agent (e.g. Dabigatran) | Inhibit factor IIa | Oral | Not advocated | Liver failure (Child’s class B and beyond)
Dose adjustment to renal function | No dose adjustment (not recommended in Child’s B/C) | Idarucizumab | Data limited to case reports and small case series (should not be used in APS) |
| Direct anti-Xa agents (e.g rivaroxaban, apixaban, edoxaban) | Inhibit factor Xa | Oral | Not advocated | Liver failure (Child’s class B and beyond)
Dose adjustment to renal function | No dose adjustment (not recommended in Child’s B/C) | Andexanet (EMA approved for rivaroxaban and apixaban) | Data limited to case reports and small case series (should not be used in APS) |
IV, intravenous; aPTT, activated partial thromboplastin tine; PT, prothrombin time; INR, international normalized ratio; EMA, European Medicines Agency; APS, antiphospholipid syndrome.
Author Biographies

Dr Dhiraj Tripathi

Dr Ashish Goel
Dr Ashish Goel is a liver speciality doctor at University Hospitals Birmingham, UK. He is currently on leave from the department of Hepatology, Christian Medical College, Vellore, India.

Dr William Lester
Dr William Lester is a Consultant Haematologist at University Hospitals Birmingham. He is a member of the Royal College of Physicians, and a fellow of the Royal College of Pathologists. His main clinical interests include thrombosis and haemostasis, von Willebrand disease, and obstetric haematology.
CME
Managing alcohol-related hepatitis and alcohol-related cirrhosis
27 February 2024
How I investigate abnormal liver function test in a patient with IBD
09 January 2024
Updates in Haemochromatosis
14 November 2023
- Intagliata NM, Caldwell SH, Tripodi A. Diagnosis, development, and treatment of portal vein thrombosis in patients with and without cirrhosis. Gastroenterology2019;156:1582–99.
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Vascular diseases of the liver. J Hepatol 2016;64:179–202.
- de Franchis R, Baveno VIF. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 2015;63:743–52.
- Yerdel MA, Gunson B, Mirza D, et al. Portal vein thrombosis in adults undergoing liver transplantation: risk factors, screening, management, and outcome. Transplantation 2000;69:1873–81.
- Sarin SK, Philips CA, Kamath PS, et al. Toward a comprehensive new classification of portal vein thrombosis in patients with cirrhosis. Gastroenterology 2016;151:574–77.e3.
- Sarin SK, Sollano JD, Chawla YK, et al. Consensus on extra-hepatic portal vein obstruction. Liver Int 2006;26:512–19.
- Tripathi D, Stanley AJ, Hayes PC, et al. U.K. guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut 2015;64:1680–704.
- Plessier A, Darwish-Murad S, Hernandez-Guerra M, et al. Acute portal vein thrombosis unrelated to cirrhosis: a prospective multicenter follow-up study. Hepatology 2010;51:210–18.
- Klinger C, Riecken B, Schmidt A, et al. Transjugular local thrombolysis with/without TIPS in patients with acute non-cirrhotic, non-malignant portal vein thrombosis. Dig Liver Dis 2017;49:1345–52.
- Benmassaoud A, AlRubaiy L, Yu D, et al. A stepwise thrombolysis regimen in the management of acute portal vein thrombosis in patients with evidence of intestinal ischaemia. Aliment Pharmacol Ther 2019;50:1049–58.
- Condat B, Pessione F, Hillaire S, et al. Current outcome of portal vein thrombosis in adults: risk and benefit of anticoagulant therapy. Gastroenterology 2001;120:490–97.
- Loffredo L, Pastori D, Farcomeni A, Violi F. Effects of anticoagulants in patients with cirrhosis and portal vein thrombosis: a systematic review and meta-analysis. Gastroenterology 2017;153:480–87.e1.
- Villa E, Cammà C, Marietta M, et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology 2012;143:1253–60.
- Caldeira D, Barra M, Santos AT, et al. Risk of drug-induced liver injury with the new oral anticoagulants: Systematic review and meta-analysis. Heart 2014;100:550–56.
- Nagaoki Y, Aikata H, Daijyo K, et al. Efficacy and safety of edoxaban for treatment of portal vein thrombosis following danaparoid sodium in patients with liver cirrhosis. Hepatol Res 2018;48:51–58.
- Intagliata NM, Henry ZH, Maitland H, et al. Direct oral anticoagulants in cirrhosis patients pose similar risks of bleeding when compared to traditional anticoagulation. Dig Dis Sci 2016;61:1721–27.
- Hanafy AS, Abd-Elsalam S, Dawoud MM. Randomized controlled trial of rivaroxaban versus warfarin in the management of acute non-neoplastic portal vein thrombosis. Vascul Pharmacol 2019;113:86–91.
- Nery F, Chevret S, Condat B, et al. Causes and consequences of portal vein thrombosis in 1,243 patients with cirrhosis: results of a longitudinal study. Hepatology 2015;61:660–67.