Author:
Dr Kevin Monahan, Consultant Gastroenterologist, Lead for the National Lynch Syndrome Transformation Project
Acknowledgements:
Adam Shaw, Consultant Clinical Geneticist, SE GMSA
Paul Fleming, Project Manager, SE GMSA
Laura Monje-Garcia, Lynch Syndrome Nurse, NT GMSA
What were the challenges to your service and why did you need to change?
Baseline national data from England demonstrated a lack of adherence to NICE guidelines for the diagnosis of Lynch syndrome (LS) in people with cancer. The National Lynch syndrome transformation project was established to address this gap in access to testing. Baseline data from 2019 demonstrated that only 43% of colorectal cancer patients received any form of index tumour ‘mismatch repair ‘(MMR) testing, and of these only 28% of eligible patients received constitutional testing for LS1. This national QI project aimed to deliver universal testing of newly diagnosed colorectal (CRC) and endometrial cancer (EC) for LS with the development of a coordinated ‘mainstreaming’ genetic testing workforce amongst cancer multidisciplinary teams (MDTs) nationally. We can now report outcomes from this national quality improvement programme.
How did you overcome the challenges?
In June 2021 regional LS project teams were established in each of 7 regional Genomic Medical Service Alliances (GMSAs), consisting of a LS doctor, nurse and project manager. A ‘Lynch syndrome champion’ was appointed within >95% of 280 local cancer MDTs, and regional teams use high quality data to develop tailored quality improvement plans for each cancer team, and multiple level training programmes. Data analysis performed by the National Disease Registration Service (NDRS) provides comprehensive diagnostic and clinicopathological data for all cancer patients diagnosed across England (total >44,000 cancers annually), including somatic and constitutional testing outcomes. In 2023, we performed an audit of consecutive patients from each cancer team nationally, to identify testing rates. A comprehensive national registry of people diagnosed with LS was developed in coordination with NDRS, to ascertain individuals for interventions, in particular for the new national LS Bowel Cancer Screening Programme (LS-BCSP) which launched in July 2023.
How did you overcome the challenges?
In June 2021 regional LS project teams were established in each of 7 regional Genomic Medical Service Alliances (GMSAs), consisting of a LS doctor, nurse and project manager. A ‘Lynch syndrome champion’ was appointed within >95% of 280 local cancer MDTs, and regional teams use high quality data to develop tailored quality improvement plans for each cancer team, and multiple level training programmes. Data analysis performed by the National Disease Registration Service (NDRS) provides comprehensive diagnostic and clinicopathological data for all cancer patients diagnosed across England (total >44,000 cancers annually), including somatic and constitutional testing outcomes. In 2023, we performed an audit of consecutive patients from each cancer team nationally, to identify testing rates. A comprehensive national registry of people diagnosed with LS was developed in coordination with NDRS, to ascertain individuals for interventions, in particular for the new national LS Bowel Cancer Screening Programme (LS-BCSP) which launched in July 2023.
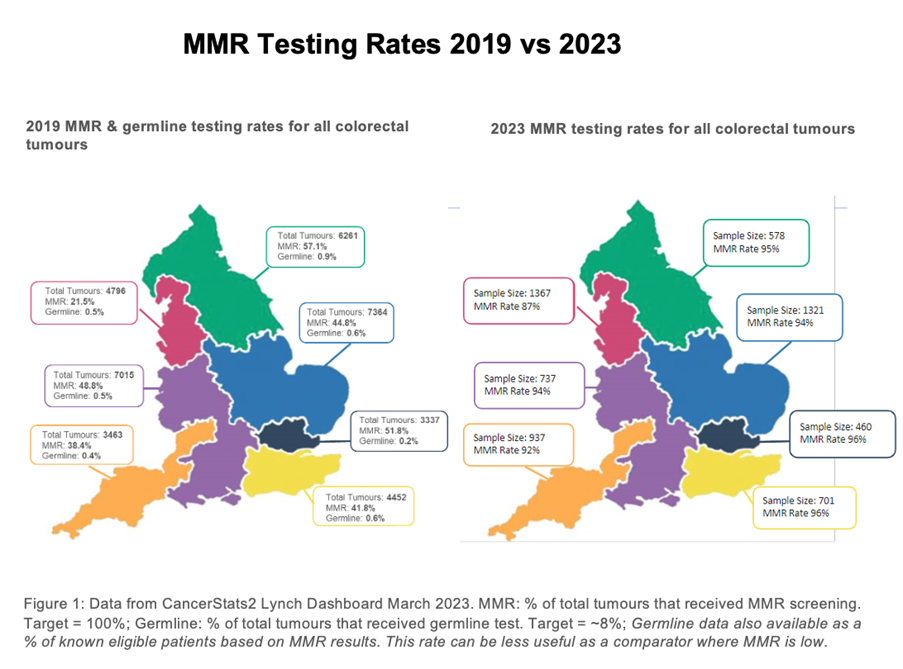
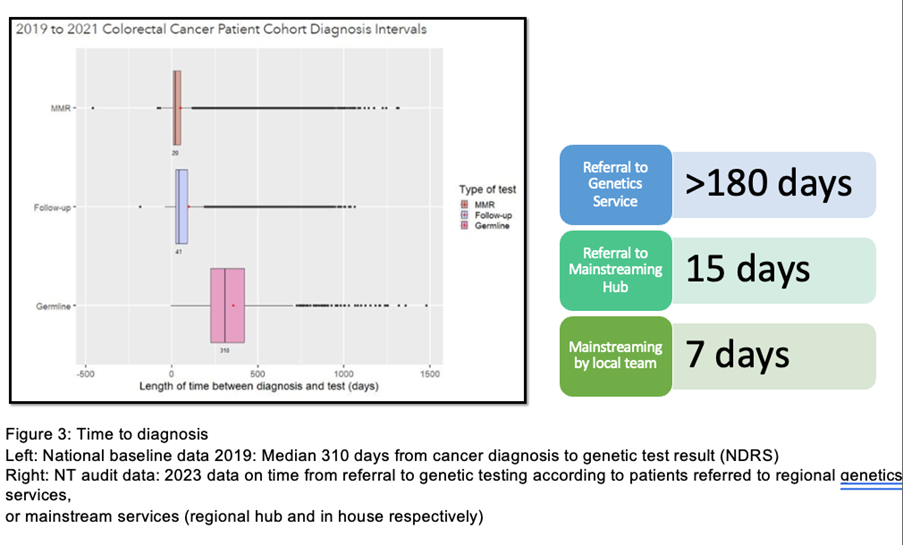
What were the learning points and how can this influence other teams?
Through the National LS Transformation project nationwide improvements in somatic and mainstreamed constitutional MMR testing for LS has been implemented, facilitating systematic delivery of universal testing for LS nationally, with equity of access to testing across all geographies. Project transformation relied on development of comprehensive data resources, identification of eligible patients, overcoming barriers, and ‘mainstreamed’ genetic testing by cancer teams. This is the largest national mainstreaming project in the world. The English National LS Transformation Project continues to evolve and embed genomics throughout cancer MDTs in England, with the establishment of new mainstreaming teams, improved access to diagnostic testing for LS in cancer patients and delivery of NICE guidelines DG27 and DG42, and the long-term outcomes of this project will be reported in a future publication. This ongoing transformational project was strongly supported by stakeholders in England. Significant quality improvement has been implemented, facilitating systematic delivery of universal testing for LS nationally, and reduction in variation in care.
Read More

