Jan Bornschein MD1,2
1 Medical Research Council Translational Immune Discovery Unit, MRC Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Headington, Oxford OX3 9DS
2 Nuffield Department of Medicine, University of Oxford, John Radcliffe Hospital, Headington, Oxford OX3 9DS
Conflict of interest: Consultancy fee from Mayoly Spindler laboratories.
Introduction
H. pylori infection is an infectious disease which has the potential to cause severe complications including peptic ulceration and gastric cancer[1]. The most common route of transmission is from parent to child in infanthood when the immune system is not yet fully matured, then usually resulting in lifelong infection. All infected individuals develop chronic-active inflammation of the stomach, but not all develop symptoms or the above mentioned complications. Most often, patients present with dyspepsia, including symptoms such as nausea, upper abdominal discomfort, low appetite or indigestion. A recent national BSG guideline gives an excellent overview on this topic[2]. Non-ulcer dyspepsia is classified either as H. pylori induced dyspepsia or non-H. pylori (functional) dyspepsia as defined in the Kyoto consensus report[1]. Thus, test-and-treat of H. pylori should always be the first step when investigating dyspepsia, since eradication allows a cure for those who are H. pylori positive.
Clinical presentation
A 21 year-old female patient was referred by her GP for investigation of upper abdominal pain that started 10 months earlier. This was mostly focused over the epigastric area and the left upper quadrant and is often accompanied by nausea, rarely leading to vomiting. She described minor weight loss. Of note is an additional diagnosis of long-standing mild iron deficiency anaemia. Initially, she was prescribed a course of PPI, which did not lead to adequate symptom improvement. The patient was then tested for H. pylori by serology (GP) and prescribed eradication treatment due to a positive result (Amoxicillin/Metronidazole/Omeprazole, 7 days). PPI treatment was continued at this point. Eradication treatment led to improvement of her symptoms, but symptom resolution was incomplete and the patient was referred to the general gastroenterology clinic at the local tertiary referral centre for further management. Bowel habits were regular apart from intermittent mild constipation. The patient was a non-smoker, did not drink alcohol and abstained from caffeinated drinks (not tolerated).
Investigations and management
An OGD was arranged to investigate persistent, PPI refractory, upper abdominal pain and nausea after H. pylori treatment, as well as to obtain biopsies to further characterize the anaemia[2,3]. The patient was asked to stop PPI intake two weeks prior to the procedure. Biopsies confirmed a persistent H. pylori infection without structural mucosal changes, and second line eradication treatment was prescribed (Amoxicillin/Clarithromycin/Omeprazole, 7 days)[4]. The patient felt substantially better and did not need any further PPI at this point. However, symptoms recurred after 2 months, mostly pain, and she started PPI intake again (Lansoprazole). A 13C-urea breath test confirmed further H. pylori persistence at this stage. Due to failure of second line treatment a further gastroscopy was arranged for biopsy based culture and resistance testing [3,4], with the results showing resistance to Clarithromycin and Levofloxacin. Third line treatment was given (Metronidazole/Tetracycline/bismuth subsalicylate/Omeprazole, 14 days)[3,4]. Again, there was symptom improvement for several weeks before symptoms recurred and further persistence was confirmed by 13C-urea breath test. Empiric fourth line treatment was given (Amoxicillin – high dose, 1000mg QDS/Rifabutin/Esomeprazole, 10 days) (expert advice, based on [5]). A breath test then confirmed successful eradication. Symptoms improved significantly, but some left upper abdominal discomfort remained.
Discussion
The general decline in incidence of both H. pylori and gastric cancer in the UK does not mean the problem is solved[6,7]. H. pylori infection can still cause significant complications and effective treatment needs to be provided. In the UK, there are two key issues in which National guidance lacks behind our European neighbours: (a) the recommended standard eradication treatment remains clarithromycin-based triple therapy for 7 days, and (b) the assessment of treatment success should be based on symptom response[4]. As seen in the patient described above, the key problem with the latter is that H. pylori eradication treatment may lead to transient symptom reduction because of the acid suppressant component in eradication regimens or even a placebo effect. Thus, some patients might be inappropriately considered as ‘cured’, and still be at risk of developing future complications. On the other hand, the clinical response in H pylori associated functional dyspepsia may take a considerably longer time, and the success of the treatment regarding symptom relief can only be assessed after a 6-12 months period (Figure 1). Furthermore, if the presenting upper GI symptoms are not primarily caused by H. pylori infection, treatment might not have an impact at all, even if eradication is achieved. This emphasizes the importance of thorough assessment of the patient’s history. Often, a clear distinction between symptom groups is not made, for example referring to patients with reflux or gallstone-induced symptoms as ‘dyspeptic’. Due to a lack of response to eradication treatment, these patients are then labelled as ‘treatment failure’ and might be prescribed further unnecessary courses of empiric antibiotics. This can generate strains of resistant bacteria which is a tremendous concern with regards to antibiotic stewardship[8].
During the last two decades, major problems have arisen due to increasing resistance rates to the antibiotics that are usually prescribed for first line eradication, including Clarithromycin, Metronidazole and Levofloxacin[9,10]. This has led to a substantial increase in first line treatment failure and the need to modify traditional treatment regimens and algorithms accordingly[3]. Unfortunately, recent data on the local and regional efficacy of eradication treatment and on resistance rates are not available for the UK[9,10]. Thus, we do not have precise results for the UK regarding the success of currently used first line regimens. The lack of these data is probably the reason why the UK guidelines on H. pylori management have not been revised recently, with the need for an update being less obvious. This led to the UK again trailing behind our continental neighbours. The recent European guidelines recommend Bismuth-based quadruple regimens as first line standard, but the national supply shortages for Bismuth pose a significant problem here. A triple therapy containing Amoxicillin, Metronidazole and PPI should be considered if there are concerns regarding regional Clarithromycin resistance or in patients who experienced adverse effects to macrolide antibiotics before. In addition, it is generally recommended to extent treatment regimens to 14 days for triple therapy, 10-14 days for quadruple regimens, with longer treatment duration showing significantly better efficacy [3, 10, 11]. In the UK, this should be considered at least for all treatment attempts after first line failure. Similarly, high dose PPI treatment should be given since appropriate acid suppression can compensate for some effects of antibiotics resistance and reduce the risk of treatment failure [10, 12]. A recent study published by the European H. pylori Registry (HpEuReg) described common pitfalls in H. pylori management, including repeat prescription of failed eradication regimens, confounders not being considered when patients are tested (e.g. test done on PPI or after recent antibiotic treatment) or treated (e.g. compliance and adherence to the prescribed regimen, impact of smoking, history of recent or previous antibiotic treatment)[13]. Due to these problems, treatment success should always be checked by appropriate, non-invasive methods after each round of treatment[8]. This includes breath testing and stool antigen testing. Serology must not be used for confirmation of treatment success due to antibody titres remaining high for years. For the same reason, serology testing should not be used as general testing tool in the UK, and only be reserved for very specific clinical scenarios (e.g. peptic ulcer bleed on PPI, when other test methods are not feasible). Furthermore, specialist referral should be considered after second line treatment failure.
Conclusion
H. pylori remains a clinical problem and should always be checked upon first presentation in patients with dyspeptic symptoms. Endoscopy is needed in first instance if the patient presents with alarm symptoms (e.g. weight loss, signs of GI bleeding), especially when in a specific age group. In the absence of Bismuth and related formulations in the UK, Clarithromycin-based triple therapy remains the standard for first line treatment. However, this should be given for 14 days as significantly better eradication rates have been reported. Furthermore, prescribers should be aware that high dose PPI should be considered since the level of acid suppression has a direct effect on treatment success (Figure 2). Referral to specialist services should be considered after second line failure, or even after first line failure in patient allergic to penicillin. Symptomatic response alone is not a reliable indicator for treatment success and patients should always proactively checked for successful eradication by non-invasive test methods to prevent long-term complications in case of H. pylori persistence.
Learning points
- Early testing for H. pylori infection should be considered in all patients with dyspeptic symptoms. Careful assessment of the patient’s history, including previous treatment attempts, facilitates further management.
- Biopsy based tests (histology, IHC, PCR, CLO), 13C-urea breath tests and stool testing must be undertaken in the absence of PPI intake for at least two weeks to avoid false negative results. Biopsies should always be taken from both antrum and body (different colonisation in patients on/off PPI, characterisation of background gastritis).
- Adherence to treatment should be checked. High dose PPI (e.g. Omeprazole 40mg BD) enhances eradication outcome. Treatment success should always be confirmed by non-invasive test methods.

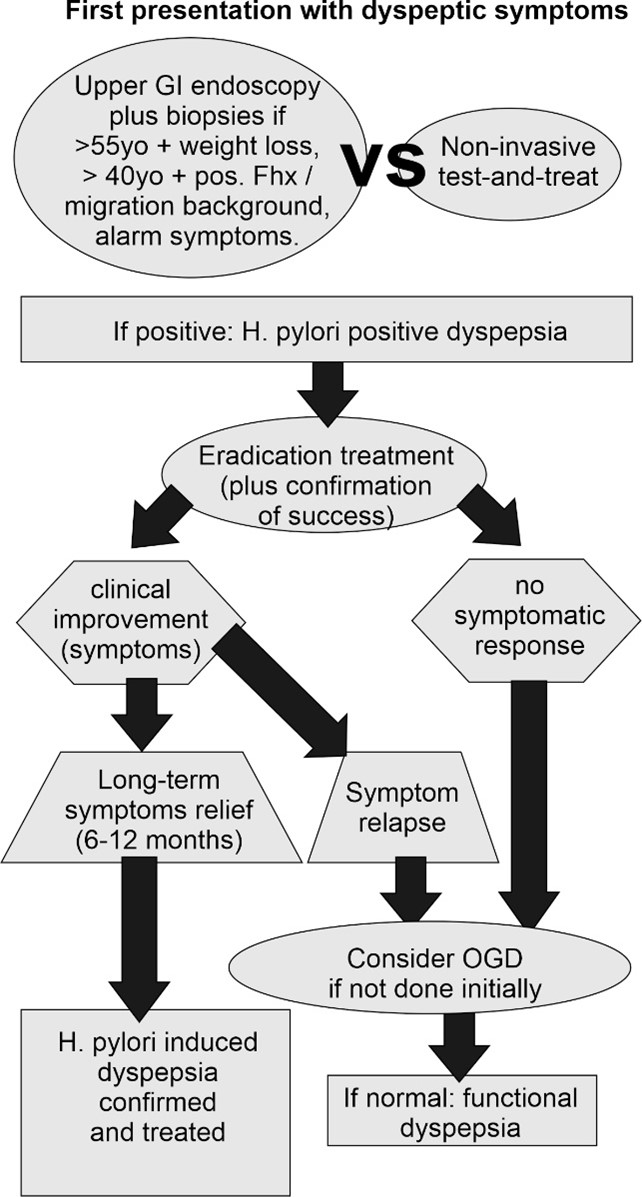
Figure 1: Proposed diagnostic algorithm
Patient with dyspeptic symptoms should initially be screened for factors that require further assessment by endoscopic tests. Otherwise, non-invasive test-and-treat of H. pylori should be performed. Success of eradication treatment should always be checked. Sustained treatment response to successful H. pylori treatment confirms H. pylori induced dyspepsia. OGD: oesophagogastroduodenoscopy; (adapted from [1]).
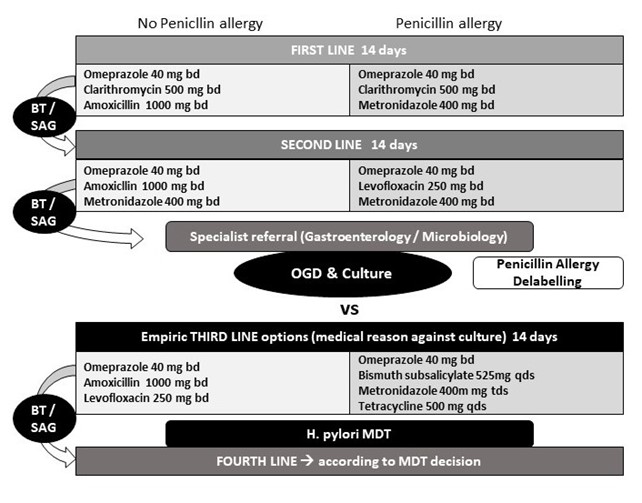

Figure 2: Proposed treatment algorithm
Treatment success should always be confirmed by non-invasive testing. Please consider treatment for 14 days already for first line regimens. High dose PPI is more efficient than standard dose with regards to eradication success rates. H. pylori culture and resistance testing should be considered after failure of second line treatment (or even after a first line treatment failure in penicillin-allergic patients). BT: breath test, MDT: multidisciplinary team meeting (involving gastroenterology, microbiology, infectious diseases and pharmacology), OGD: oesophagogastroduodenoscopy, SAG: stool antigen test.
Acknowledgments
JB is supported by the UK Medical research Council in context of the Clinical Academic Research Partnership (MRC CARP) scheme (Grant ref.: MR/W029960/1).
Author Biography

Jan Bornschein is a consultant gastroenterologist at Oxford University Hospitals NHSFT, and Honorary Senior Clinical Lecturer at the Nuffield Department of Experimental Medicine, University of Oxford. He trained as Gastroenterologist in Magdeburg, Germany, before joining the MRC Cancer Unit, University of Cambridge, for a Post Doc project in 2013. His main clinical interest is in gastrointestinal oncology as well as upper GI disease in general. His research focus lies on inflammation-induced carcinogenesis in the upper GI tract. He was involved in the last 3 editions of both the German guidelines on H. pylori and ulcer disease as well as diagnosis and management of gastric cancer. He currently represents the BSG on a guideline panel addressing the management of side effects of cancer treatment. He is an active member of the BSG (Gastroduodenal section committee) and the German Society of gastroenterology (section GI oncology), board member of the EAGEN, and he serves as representative for general gastroenterology on the UEG council.
CME
NSAID induced gastrointestinal damage
11 December 2023
4 points
Masterclass: Gastroparesis
01 November 2023
1 Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015;64:1353–67. doi:10.1136/gutjnl-2015-309252
2 Black CJ, Paine PA, Agrawal A, et al. British Society of Gastroenterology guidelines on the management of functional dyspepsia. Gut 2022;71:1697–723. doi:10.1136/gutjnl-2022-327737
3 Malfertheiner P, Megraud F, Rokkas T, et al. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut Published Online First: 1 September 2022. doi:10.1136/gutjnl-2022-327745
4 McNulty C. Test and treat for Helicobacter pylori (HP) in dyspepsia. Quick reference guide for primary care: For consultation and local adaptation. 2017.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/828593/HP_Quick_Reference_Guide_v18.0_August_2019_change_highlighted.pdf
5 Nyssen OP, Vaira D, Saracino IM, et al. Experience with Rifabutin-Containing Therapy in 500 Patients from the European Registry on Helicobacter pylori Management (Hp-EuReg). J Clin Med 2022;11. doi:10.3390/jcm11061658
6 Hooi JKY, Lai WY, Ng WK, et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017;153:420–9. doi:10.1053/j.gastro.2017.04.022
7 Zamani M, Ebrahimtabar F, Zamani V, et al. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther 2018;47:868–76. doi:10.1111/apt.14561
8 Bornschein J, Pritchard DM. Myths and misconceptions in the management of Helicobacter pylori infection. Frontline Gastroenterol 2022;13:245–53. doi:10.1136/flgastro-2021-101826
9 Megraud F, Bruyndonckx R, Coenen S, et al. Helicobacter pylori resistance to antibiotics in Europe in 2018 and its relationship to antibiotic consumption in the community. Gut 2021;70:1815–22. doi:10.1136/gutjnl-2021-324032
10 Nyssen OP, Bordin D, Tepes B, et al. European Registry on Helicobacter pylori management (Hp-EuReg): Patterns and trends in first-line empirical eradication prescription and outcomes of 5 years and 21 533 patients. Gut 2021;70:40–54. doi:10.1136/gutjnl-2020-321372
11 Puig I, Baylina M, Sanchez-Delgado J, et al. Systematic review and meta-analysis: triple therapy combining a proton-pump inhibitor, amoxicillin and metronidazole for Helicobacter pylori first-line treatment. J Antimicrob Chemother 2016; 71:2740-53. doi: 10.1093/jac/dkw220.
12 Villoria A, Garcia P, Calvet X, et al. Meta-analysis: high-dose proton pump inhibitors vs. standard dose in triple therapy for Helicobacter pylori eradication. Aliment Pharmacol Ther 2008;28:868-77.
doi: 10.1111/j.1365-2036.2008.03807.x
13 Nyssen OP, Vaira D, Tepes B, et al. Room for Improvement in the Treatment of Helicobacter pylori Infection: Lessons from the European Registry on H. pylori Management (Hp-EuReg). J Clin Gastroenterol 2022;56:E98–108. doi:10.1097/MCG.0000000000001482
